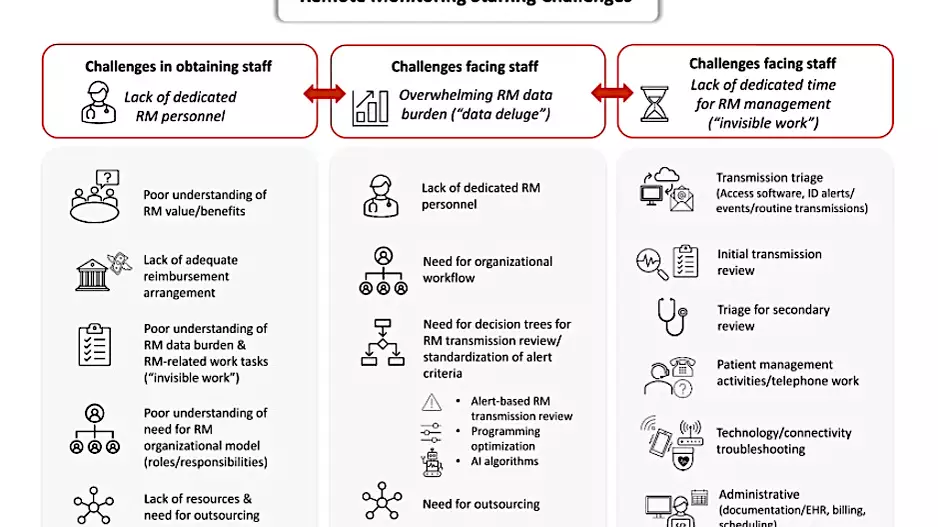
More Articles
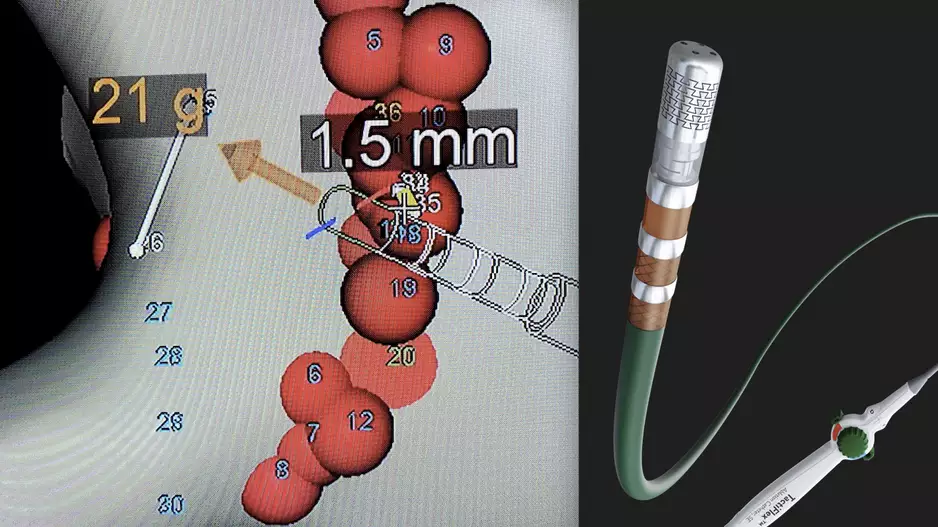
The U.S. Food and Drug Administration (FDA) recently cleared Abbott's TactiFlex Ablation Catheter, Sensor Enabled, which is the the world's first ablation catheter with a flexible tip and contact force technology. The radiofrequency (RF) catheter…
‘Notably high’ rates of PTSD, depression and anxiety seen in patients with implantable heart devices
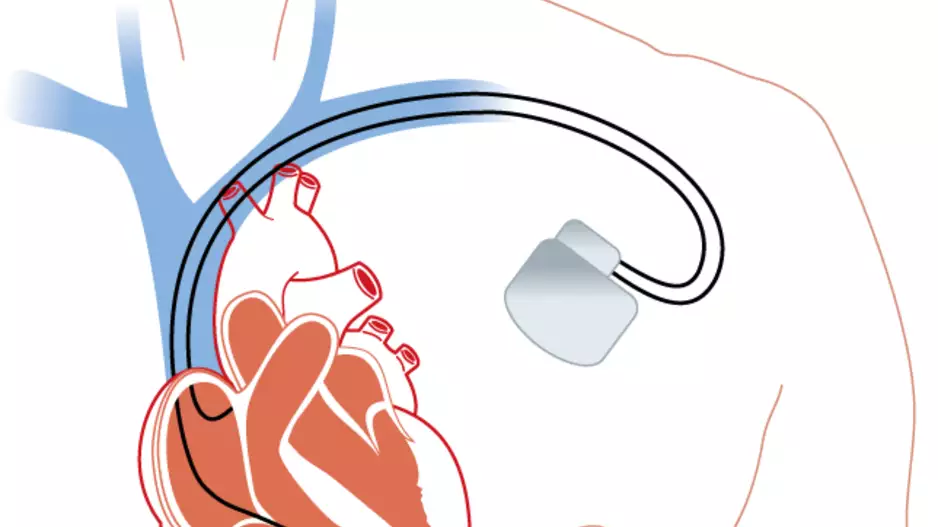
Post-traumatic stress disorder (PTSD), depression and anxiety are all much more common among patients who receive an implantable cardioverter defibrillator (ICD) than the general population,…
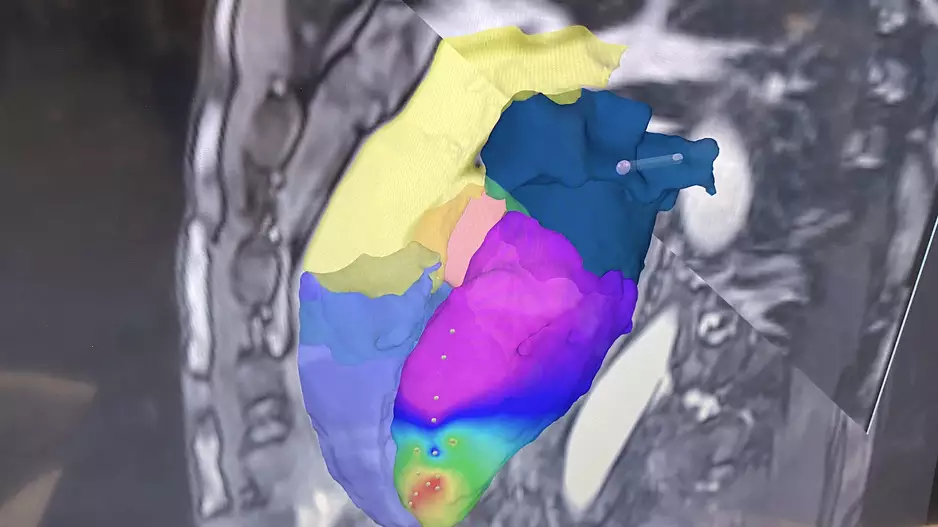
One of the biggest technology trends in cardiac electrophysiology is the the development of pulsed field ablation (PFA), a non-thermal ablation treatment for patients with atrial fibrillation (AF). The Heart…
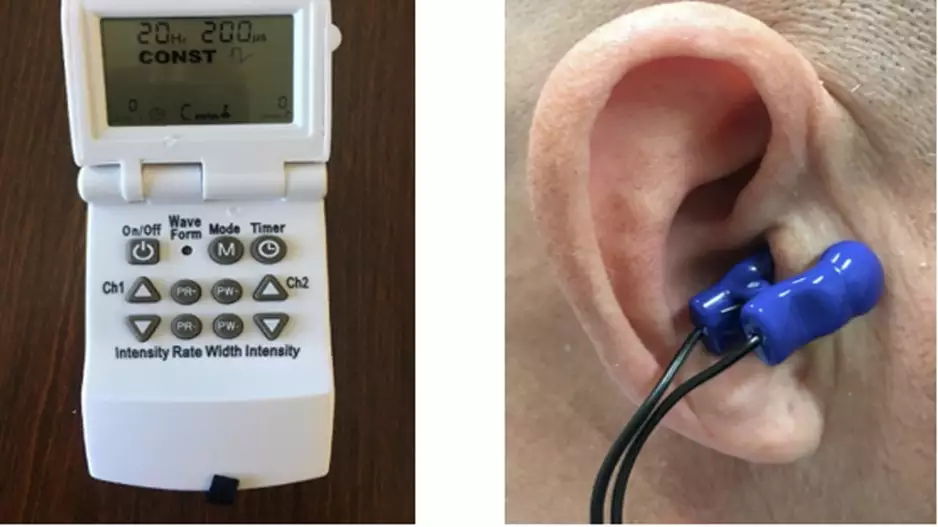
A patient-administered therapy ear clip device effectively uses nerve stimulation to treat postural tachycardia syndrome (POTS), according to a late-breaking clinical trial at Heart Rhythm 2023 [1]. This may offer a new treatment option for…
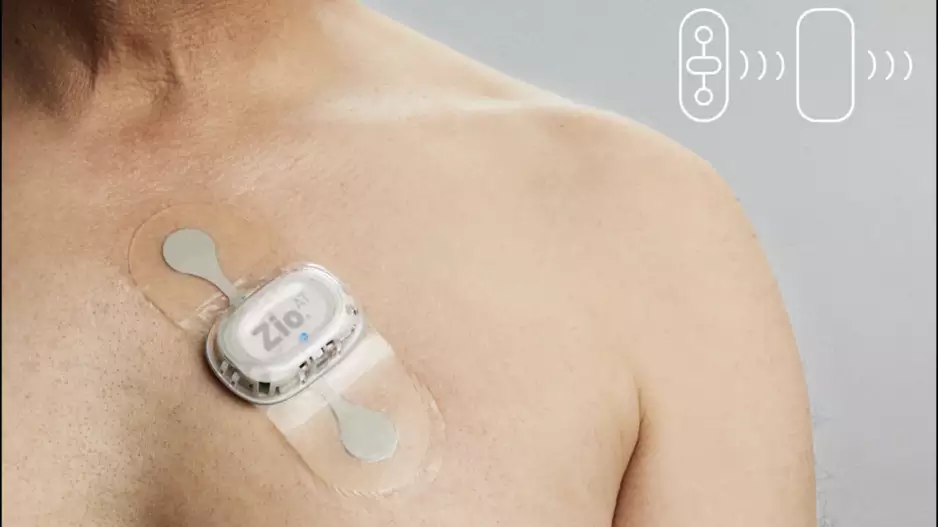
The U.S. Food and Drug Administration (FDA) sent a warning letter to iRhythm Technologies detailing several issues related to the company’s Zio AT mobile cardiac telemetry device. The letter was sent…
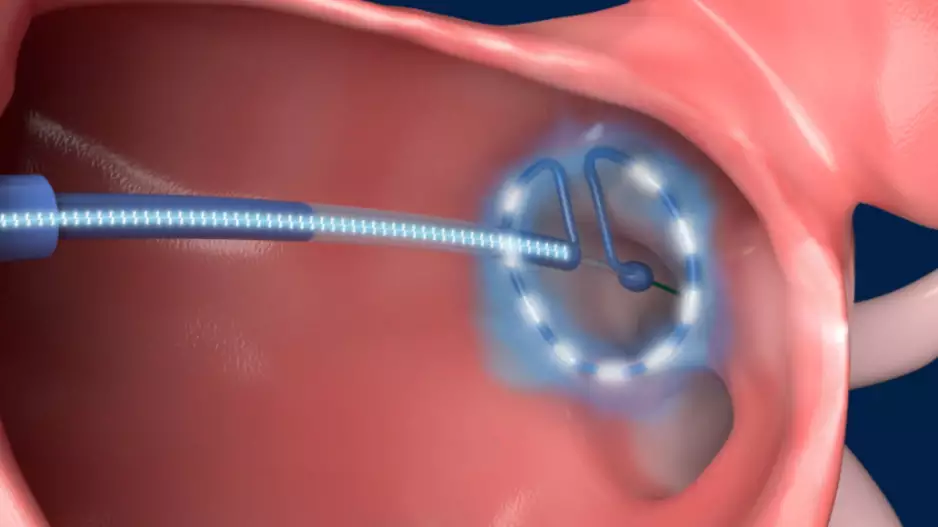
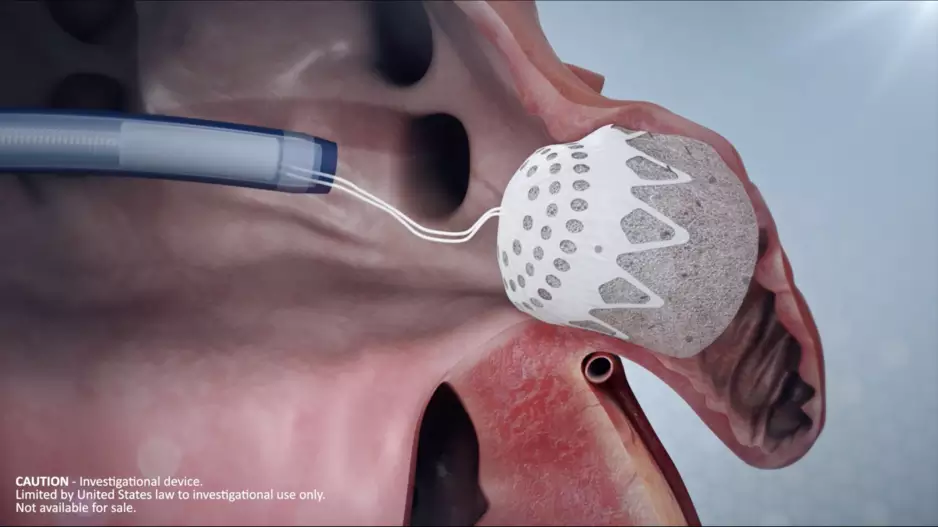
Conformal Medical, a New Hampshire-based medical device company founded in 2016, has completed a Series D funding round worth $35 million. The new investments come as the company continues developing its…
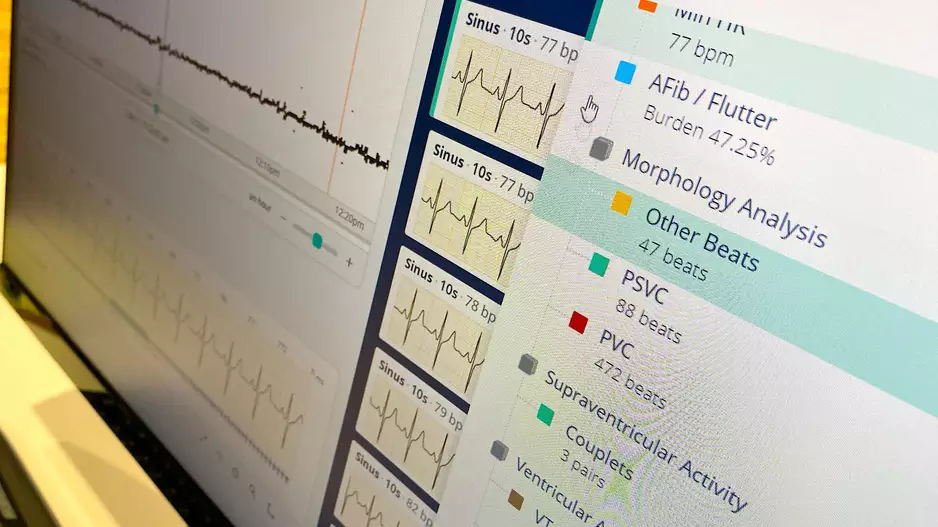
An artificial intelligence (AI) algorithm that analyses electrocardiograms…
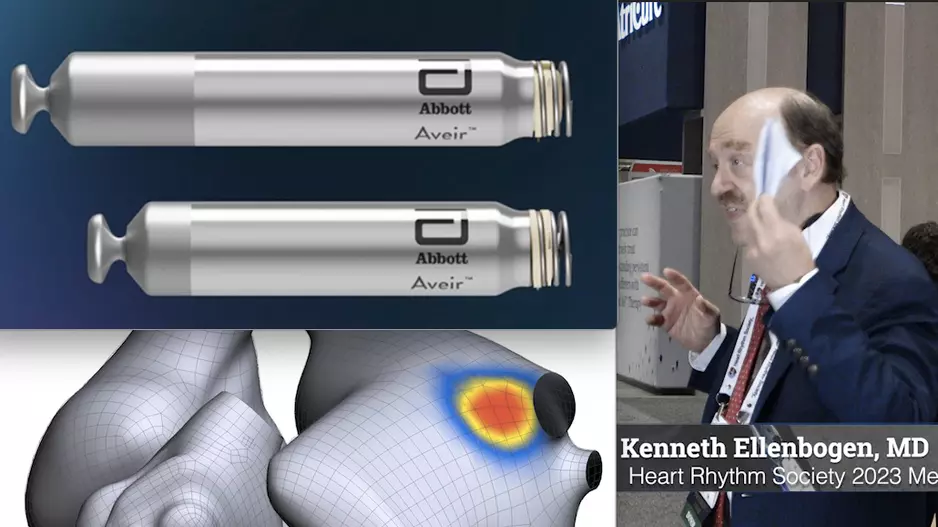
Results from a pivotal clinical trial presented as a late-breaker at Heart Rhythm 2023 indicate that a new leadless…
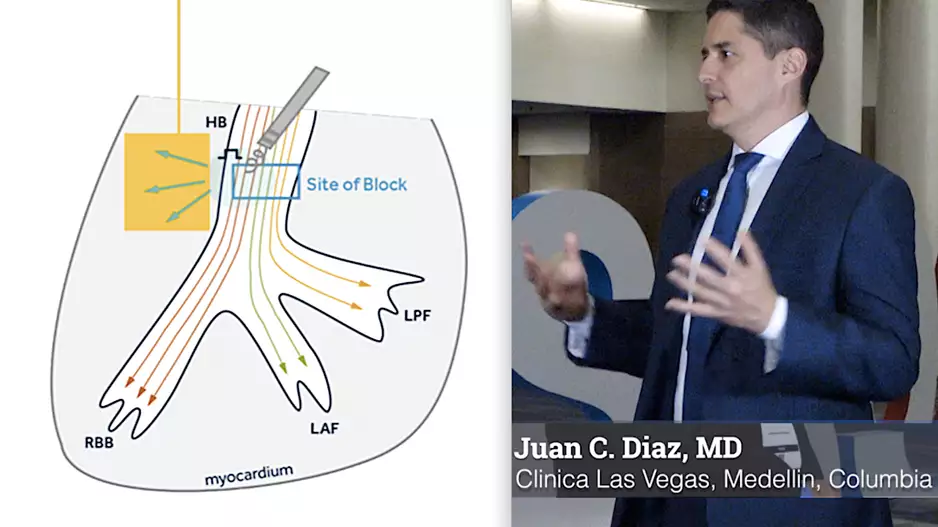
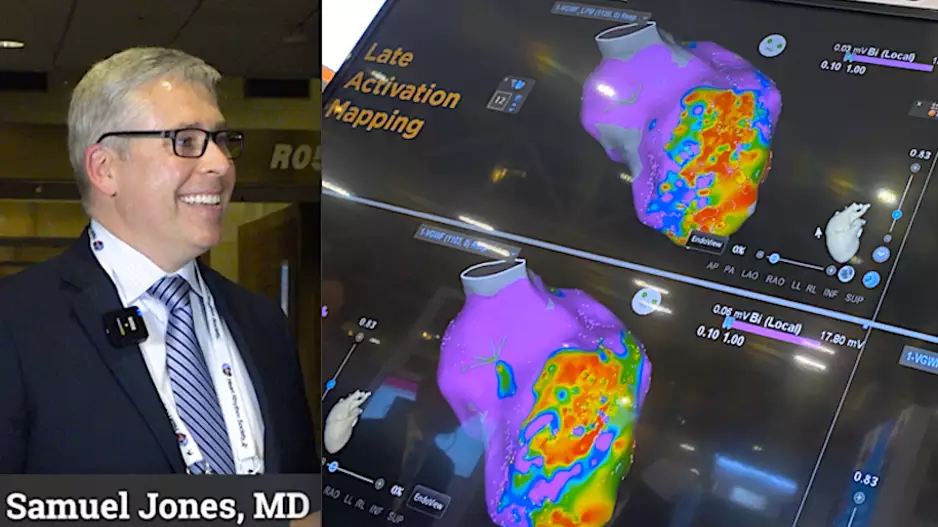
Two late-breaking clinical studies at Heart Rhythm 2023 highlight the success of left bundle branch area pacing (…
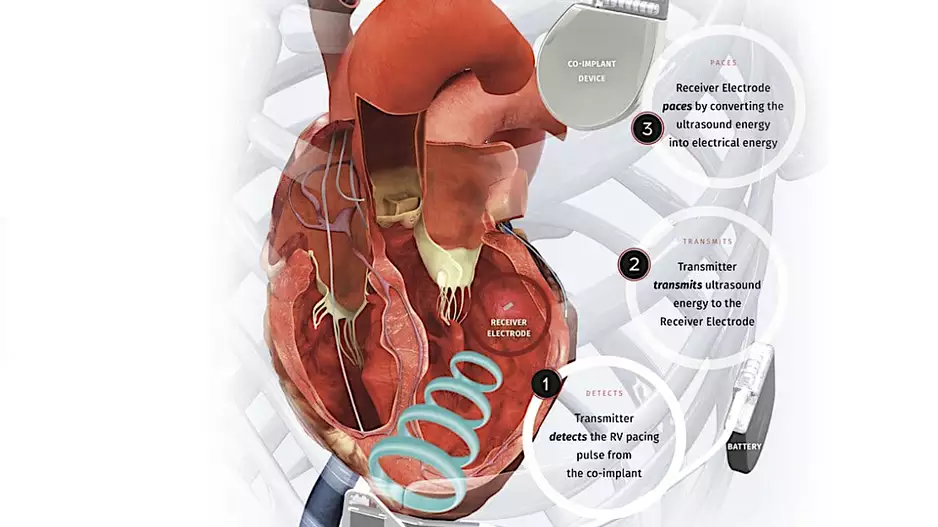
Two veteran cardiologists from Cleveland Clinic have performed the first heart procedure of its kind, successfully implanting a new dual cardiac device in a patient presenting with heart failure symptoms.
…
Abbott has received U.S. Food and Drug Administration (FDA) approval for its Assert-IQ insertable cardiac monitor (ICM). The device, designed for the long-term monitoring of arrhythmias, uses…
Asundexian, a new investigational drug from Bayer, is now one step closer to being approved by the U.S. Food and Drug Administration (FDA) to treat atrial fibrillation (AFib).
…