Cath Lab
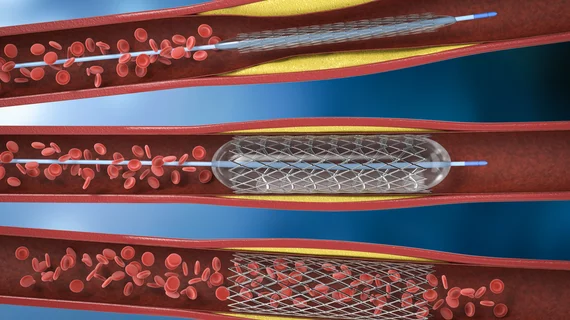
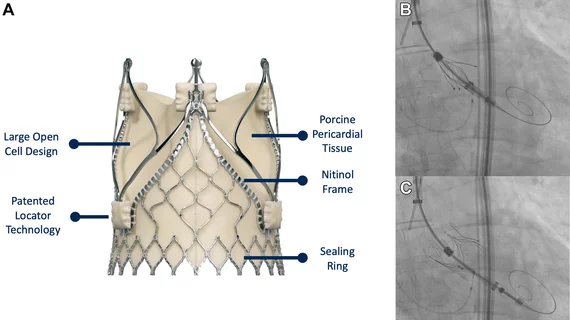
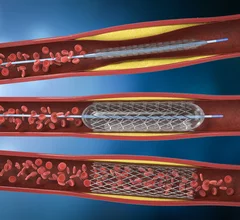
The cardiac catheterization laboratory is used for diagnostic angiograms and percutaneous coronary interventions (PCI). Cath labs have also seen expanding use in recent years for transcatheter structural heart procedures. Some hospitals also share these labs with other subspecialties for catheter-based procedures in electrophysiology (EP), interventional radiology, peripheral artery disease (PAD), carotid and neuro interventional procedures and vascular surgery.

Displaying 1 - 8 of 456