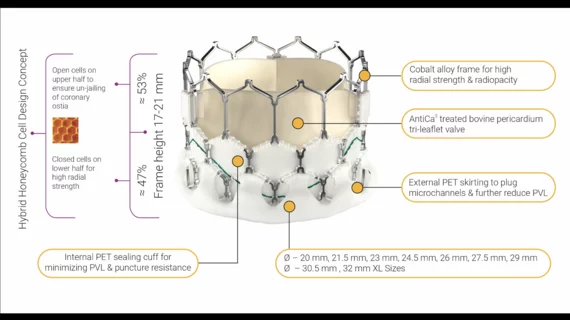
Structural Heart Disease
Structural heart diseases include any issues preventing normal cardiovascular function due to damage or alteration to the anatomical components of the heart. This is caused by aging, advanced atherosclerosis, calcification, tissue degeneration, congenital heart defects and heart failure. The most commonly treated areas are the heart valves, in particular the mitral and aortic valves. These can be replaced through open heart surgery or using cath lab-based transcatheter valves or repairs to eliminate regurgitation due to faulty valve leaflets. This includes transcatheter aortic valve replacement (TAVR). Other common procedures include left atrial appendage (LAA) occlusion and closing congenital holes in the heart, such as PFO and ASD. A growing area includes transcatheter mitral repair or replacement and transcatheter tricuspid valve repair and replacement.