More Articles
The American Stroke Association plans on honoring nine scientists and stroke researchers at its annual International…
Researchers and others in the healthcare industry are gathering this week in Los Angeles for the annual American Heart Association/American Stroke Association international stroke conference.
Kyra J. Becker, MD, FAHA, the conference…
Cook Medical voluntarily recalled 360 lots of its single lumen central venous catheters and pressure monitoring sets and trays, according to an FDA news release. The recall…
The FDA granted Spectranetics 510(k) clearance on Feb. 8 for its bridge occlusion balloon for temporary vessel occlusion in cardiac lead extraction procedures.
Spectranetics …
The American College of Cardiology (ACC) has announced that it is partnering with the Chinese Society of Cardiology to launch a cardiovascular disease education and awareness program in China.
The program, which is supported by Pfizer, will…
On Jan. 7, the U.S. Department of Health and Human Services (HHS) and U.S. Department of Agriculture (USDA) released updated dietary guidelines to improve health and help prevent chronic disease.
However, Steven E. Nissen, MD, of the…
The FDA recalled the catheter included in the Fuhrman Pleural/Pneumopericardial Drainage Set, which is used to remove air from the pericardium surrounding the heart or drain fluid from the pleural cavity that protects the lungs.
The class I…
The American College of Cardiology (ACC) launched a registry on Dec. 16 to track data on left atrial appendage occlusion (LAAO) devices.
The …
The FDA approved the AngioJet ZelanteDVT thrombectomy catheter on Nov. 30 to treat patients with deep vein thrombosis. The catheter also received a CE Mark on the same day for approval in Europe.
The ZelanteDVT catheter offers four times…
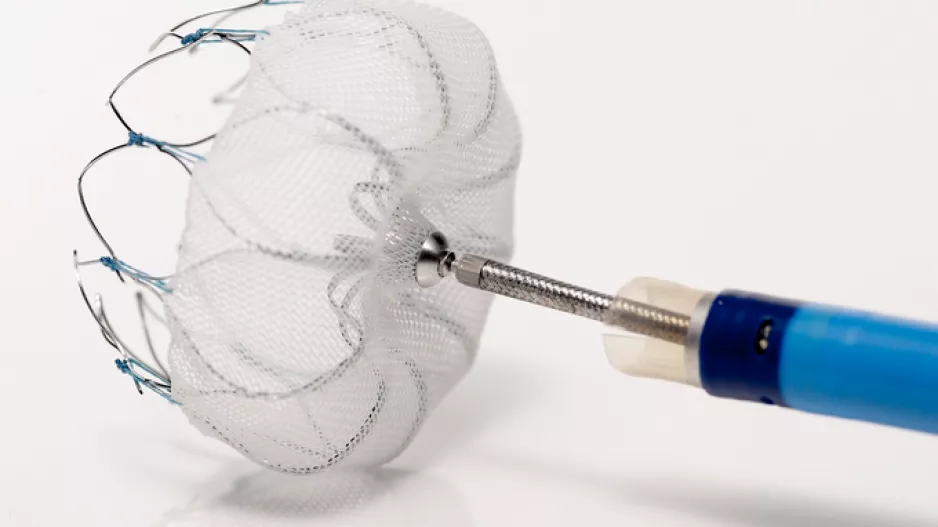
After 30 days of follow-up, 92.7 percent of patients with atrial fibrillation at high-risk for stroke who were implanted with the Watchman device did not have any serious adverse events, according to an analysis of an international registry. In…
Medtronic Unveils New Aortic and Peripheral Data from Two Late-Breaking Clinical Trials at VIVA 2015
Medtronic announced new clinical data in interventional treatments for aortic and peripheral vascular diseases in a late-breaking trial session at Vascular Interventional Advances (VIVA) 2015.
Valiant Captiva Demonstrates Safety and…
Researchers discovered a molecule that may be a potential therapy for patients recovering from a stroke.
The molecule, known as growth and differentiation factor 10 (GDF10), is activated early after stroke, according to researchers from the…
As part of its annual Medical Innovation Summit, the Cleveland Clinic released its top 10 medical innovations for 2016. The …
Cook Medical announced that it has expanded its voluntary recall of angiographic catheters to include all lots of specific sizes.
The catheters have exhibited tip splitting or separation, resulting in the FDA receiving a total of 42 Medical…
After two years of follow up, patients with peripheral artery disease who received a drug-coated balloon had significantly higher primary patency and a significantly lower rate of clinically driven target lesion revascularization compared with…
A meta-analysis of five randomized controlled trials found that performing endovascular therapy with stent retrievers was safe and effective in managing acute ischemic stroke and led to a significant improvement in functional clinical outcomes…
The FDA approved the combination of ambrisentan and tadalafil to reduce the risks of disease progression and hospitalization for worsening pulmonary arterial hypertension (PAH) and improve exercise ability.
Ambrisentan, an endothelin…
Medtronic agreed to acquire Lazarus Effect, a medical device company with an acute ischemic stroke product that is approved in Europe but not in the U.S. The deal, which is subject to customary closing conditions, is an all-cash transaction…
Medtronic acquired Medina Medical, a medical device company developing a device to treat cerebral aneurysms. Medtronic had previously invested and held an ownership stake in Medina Medical, which is based in Menlo Park, Calif.
Medtronic…
Hospitals across the U.S. are beginning to implant patients with the Watchman left atrial appendage closure device, which the FDA …
Employees who work 55 or more hours per week had a higher risk of stroke compared with those who work standard hours, according to a cumulative random-effects meta-analysis of published and unpublished data.
Lead researcher Mika Kivimäki,…