More Articles
The FDA approved the LifeVest wearable cardioverter defibrillator (Zoll Lifecor Corp.) on Dec. 17 to treat some children who are at risk for sudden cardiac arrest. The device was approved in 2001 for adults who were at least 18 years old.
…
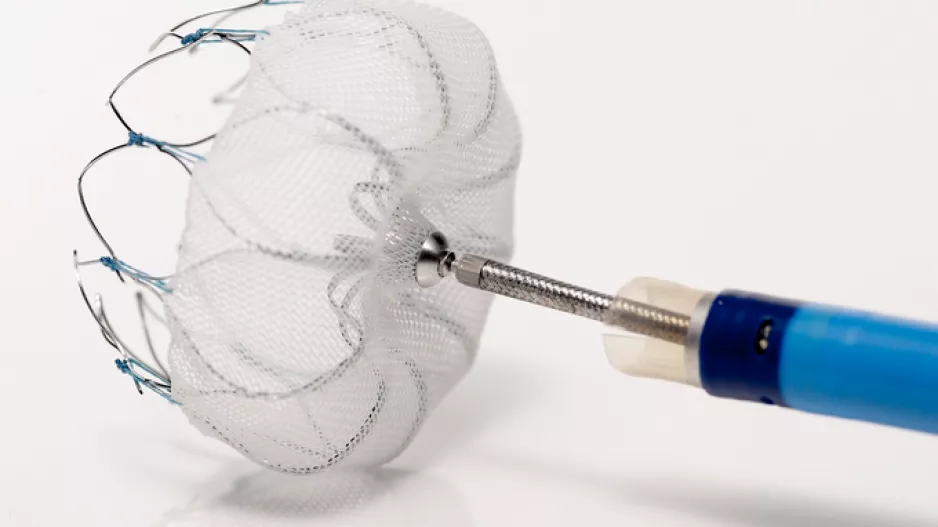
Three leading cardiology societies released a consensus statement on Dec. 10 regarding criteria institutions and operators should follow for left atrial appendage occlusion.
The…
Major electrophysiology and cardiology societies released an expert consensus statement on Nov. 19 on optimal implantable cardioverter-defibrillator (ICD) programming and testing.
The authors mentioned that programming and surgical…
Despite an FDA safety alert in July, physicians are still using the Lariat suture delivery…
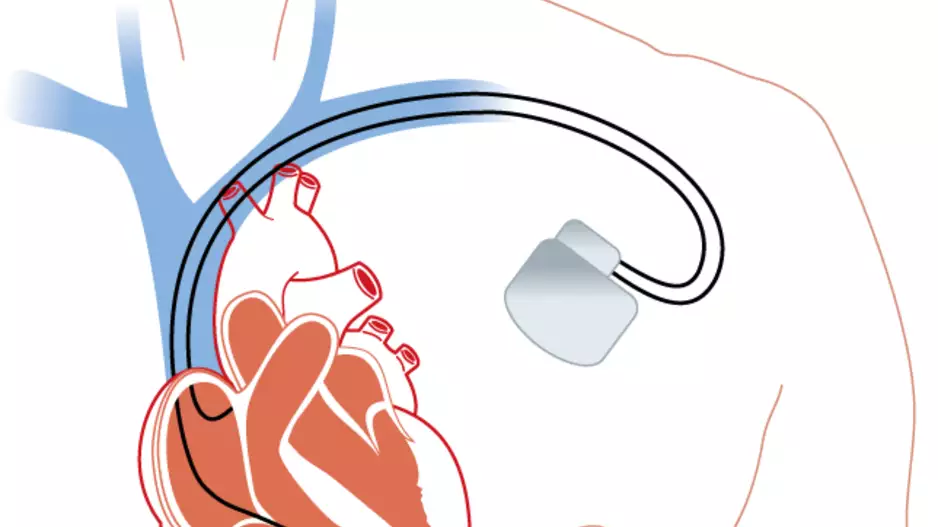
Six months after implantation with an investigational leadless intracardiac transcatheter pacing system from Medtronic, 96 percent of patients did not have system-related or procedure-related major complications, according to a prespecified…
Although the Department of Justice (DOJ) announced on Oct. 30 that it had…
Three major cardiology societies released a new guideline on Sept. 23 to manage adults with all types of supraventricular tachycardia (SVT) other than atrial fibrillation.
The American College of Cardiology (ACC), American Heart…
Three decades and 1 million-plus implantations after its introduction, the transvenous implantable cardioverter-defibrillator (T-ICD) is facing stiff competition from a smaller, thinner, more versatile and longer lasting addition to the…
The FDA approved the Evera MRI Surescan implantable cardioverter defibrillator (ICD) as the first ICD system for use with MRI scans.
The Evera MRI ICD system (Medtronic) is intended for patients with sudden cardiac arrest. The …
After six months of nonsurgical implantation with an active-fixation leadless cardiac pacemaker, 90 percent of patients had an acceptable pacing threshold and sensing amplitude, according to a prespecified analysis of an ongoing study.
In…
The American College of Cardiology (ACC) announced it plans on launching two clinical…
The Heart Rhythm Society focused on the latest developments in electrophysiology and the management of patients with rhythm disorders and heart failure at its 2015 meeting on May 13-16 in Boston. The event included 12 late-breaking clinical…
Are Riata concerns a thing of the past and transcatheter aortic valve replacement (TAVR) for almost anyone with severe aortic stenosis the way of the future? Presentations at recent cardiology conferences addressed those questions.
The…
As an early career electrophysiologist, Richard I. Fogel, MD, learned to raise his hand…
Johnson & Johnson will give outside researchers access to clinical data on diagnostics and medical devices through Yale University, but the largesse applies only to products approved in 2014 or later. That makes the ThermoCool SmartTouch…
Medtronic, Inc. (NYSE: MDT) today announced the U.S. Food and Drug Administration (FDA) approval and commercial launch of two additional Attain Performa® left ventricular (LV) quadripolar leads, which can be paired with the Medtronic…
Meet retired electrophysiologist David Mann, MD, not to be confused with all the other doctors with the same name. Except unfortunately for him, he was, and in the one of the thorniest places imaginable—the Centers for Medicare & Medicaid…
Researchers at Cedars-Sinai Heart Institute in Los Angeles tested a “biological pacemaker” in pig hearts using gene therapy. The approach is at least three years away from human trials, the New York Times reports.
Preregistration attendance for Heart Rhythm Society 2014 has increased significantly compared with last year in all demographic categories, said John D. Day, MD, chair of the scientific session program committee, with notable bumps in…
Bloomberg News reported that Boehringer Ingelheim provided the FDA with one analysis showing a lower rate of fatal bleeding events in patients treated with dabigatran (Pradaxa) than was found in a second analysis that the company chose not to…
More than 2,000 patients in the U.S. have filed suits against Boehringer Ingelheim over its oral anticoagulant dabigatran (Pradaxa). The company confirmed the number to Reuters after a German newspaper initially reported the total.