The U.S. Centers for Disease Control and Prevention (CDC) is now recommending that some younger patients wait up to eight weeks after their first dose of an FDA-approved or FDA-authorized COVID-19 vaccine. Waiting a few additional weeks, the agency wrote, could potentially help lower “the small risk of myocarditis” associated with these vaccines.
A three-week interval between doses of the Pfizer-BioNTech vaccine, and a four-week interval between doses of the Moderna vaccine, are currently recommended. Adjusting this interval, however, could help patients most likely to experience vaccine-related myocarditis avoid the side effect altogether.
“An eight-week interval may be optimal for some people ages 12 years and older, especially for males ages 12 to 39 years,” according to a new recommendation from the agency. “A shorter interval between the first and second doses remains the recommended interval for people who are moderately or severely immunocompromised, adults ages 65 years and older and others who need rapid protection due to increased concern about community transmission or risk of severe disease.”
“Age-appropriate booster doses” are still recommended for all individuals 12 years old and older. This includes patients who were 11 years old at the time of their first or second dose.
“Currently, COVID-19 vaccines are not authorized for a booster dose for children ages 11 years and younger,” the CDC emphasized.
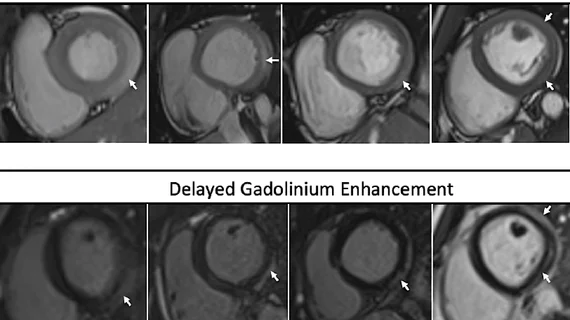
Reviewing the risk of vaccine-related myocarditis and/or pericarditis
The CDC website also provides physicians and patients with a detailed look at the “rare risk” or vaccine-related myocarditis and pericarditis. This is most commonly seen in men between the ages of 12 and 29 after they receive their second dose of an FDA-approved or FDA-authorized vaccine.
“To date, data suggest the risk for myocarditis and/or pericarditis after mRNA COVID-19 booster doses in young adults appears lower than risk after the primary mRNA COVID-19 vaccine series,” according to the CDC. “Cases of myocarditis among children aged 5–11 years after mRNA COVID-19 vaccine have been rarely reported, and it is not yet known if there is any increased risk for myocarditis or pericarditis after mRNA COVID-19 vaccination in this age group; monitoring is ongoing.”
Related COVID-19 Cardiology Content:
Cardiac MRI sheds new light on vaccine-related myocarditis
New scoring system predicts stroke risk after COVID-19
41% of Americans have experienced heart-related issues during the pandemic
4 key takeaways from an updated look at vaccine-related myocarditis in the US
Additional stories are available here.