Physicians and patients have long awaited the next step beyond catheter ablation for ventricular tachycardia (VT). Could noninvasive stereotactic body radiation be that breakthrough?
MANAGING EXPECTATIONS
From the moment the study was published in the New England Journal of Medicine this past December, the kudos began raining down from the cardiology and electrophysiology cybersphere. “Game changer.” “Paradigm shift.” “The future of arrhythmia management.” “Pivotal study in advancing science and technology.”
Indeed, this embarrassment of riches put the authors of the study, “Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia” (2017;377[24]:2325-36) in an awkward, if not enviable, position. Instead of the usual post-publication parrying to defend their work, they felt compelled to tamp down expectations. “We found ourselves saying to everyone, ‘Take a deep breath, settle down,’” recalls Clifford Robinson, MD, a radiation oncologist and associate professor at Washington University School of Medicine in St. Louis and a senior author of the study. “There is still so much to be learned here, and we want to do this carefully.”
Not that the results of their experience with five patients with high-risk refractory VT—patients who had exhausted their clinical options and were counting their life expectancy in months—weren’t cause for enthusiasm. In the three months before treatment, the patients had a combined history of 6,577 VT episodes. During the six weeks after treatment with noninvasive cardiac radioablation—a period when arrhythmias can still occur due to post-ablation inflammation—there were a total of 680 VT episodes. In the following year of intense patient tracking, these events dwindled to just four collectively, for a reduction of 99.9 percent. Two patients experienced no episodes at all.
“It was really eye-opening to see results as strong and lasting as these in patients who were at the end of the road in terms of treatment options,” says Phillip Cuculich, MD, lead author and an electrophysiologist and associate professor at Washington University School of Medicine in St. Louis. “But so many things in medicine have enjoyed early hype and when you more rigorously and prospectively test them in clinical trials, that hype doesn’t hold up. So, I think the proper words here are cautiously optimistic.”
OVERCOMING HURDLES
One obvious caveat is the small number of patients treated and the incomplete picture that may have conveyed. “I don’t know what kinds of complications might turn up if you looked at a larger group of patients,” offers Anne Curtis, MD, chair of the department of medicine at the University of Buffalo and president of the Cardiac Electrophysiology Society. She qualified that concern, however, with strong praise for the technical approach embraced by the researchers, calling it “really exciting” and “very impressive.”
Well aware of the limitations of such a small sample size, the research team was hard at work on the next phase of their study after waiting for the one-year patient results to drift in from the initial effort. This time, 19 patients were enrolled as part of the phase I/II prospective trial known as ENCORE-VT, aimed at people with VT who had failed standard therapy, including medications and invasive catheter ablation. That study, which Cuculich says captured patients at a much quicker rate than had been expected, has completed enrollment and will provide early results this summer. “We think the brisk enrollment speaks to the clinical need in this space for better, safer and faster ways to treat patients with refractory arrhythmia,” he says.
Perhaps the biggest question mark hanging over the noninvasive procedure, however, is the impact that targeting the heart with a high-dose burst of stereotactic body radiation therapy (SBRT)—a treatment initially developed to treat small brain tumors—might have on patients down the road. Admittedly, this was not a major consideration for the gravely ill patients who participated in the early SBRT studies. But what might the health implications be if the treatment were expanded to a younger, healthier cohort?
Roy John, MB, BS, PhD, a cardiac electrophysiologist and associate professor of cardiovascular medicine at Vanderbilt University Medical Center in Nashville, points out that physicians are now seeing patients with complications from radiation therapy they received 30 and even 40 years ago for lymphoma. “I would say [SBRT] is potentially a game-changing technology,” says John, who co-authored an editorial that accompanied the study published in NEJM (2017;377[24]:2388-90). “But we need to make sure that whatever we do is safe, and it’s too early in the game to say this is going to fly.”
Among those who would subscribe to that notion is Robinson, who has gone to great lengths during his career as a radiation oncologist to avoid exposing the heart to potential toxicity while treating patients for various forms of cancer. “Just as there have been concerns in the ablation world that incomplete ablations or the ablation itself might be pro-arrhythmogenic down the line, there is the possibility that over time incomplete radiation damage at the margins of where we treat could end up generating arrhythmias,” he explains.
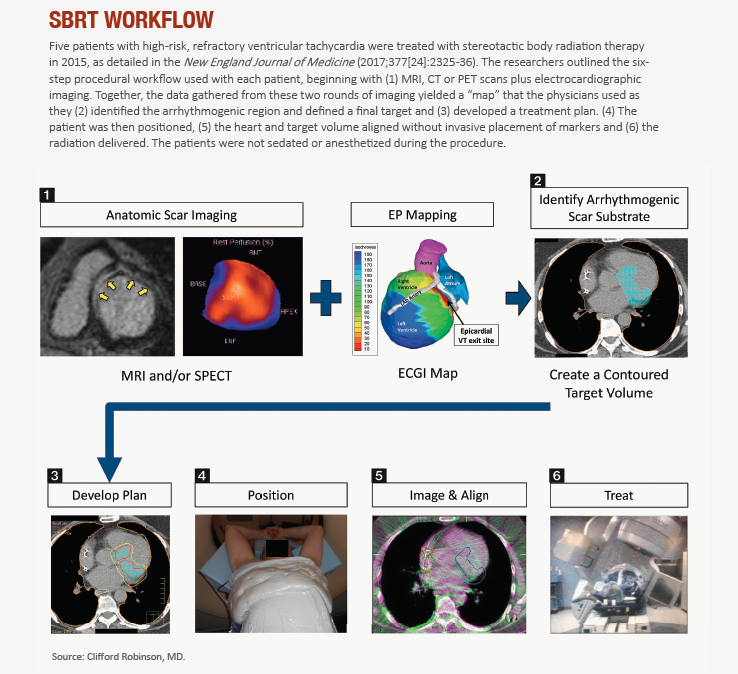
CURRENT STANDARD OF CARE
What’s not in doubt are the benefits noninvasive radioablation could hold for patients with erratic electrical signals of the heart when compared to the current standard of care. Despite antiarrhythmic drugs and advances in catheter ablation, VT claims more than 300,000 lives annually in the U.S., making it responsible for most sudden cardiac deaths. “Even after an apparently successful VT ablation, at least 30 percent of patients experience recurrence over the following year,” observes John, adding that the procedure’s effectiveness is limited by the fact that some VT substrate is difficult for physicians to locate and thus not accessible to catheters.
Therein is one of catheter ablation’s greatest liabilities: the grueling demands it puts on patients, many of them seriously ill with histories of heart attack, heart failure and various other diseases like lung and kidney, and many of them physically ill-equipped for a six- to eight-hour ablation with its accompanying thicket of breathing tubes, multiple IVs and invasive catheters. One recent retrospective study at 12 international centers of more than 2,000 patients with structural heart disease undergoing catheter ablation of VT drove home the cost of this invasiveness: 5 percent of patients died within a month of the procedure, including 3 percent before hospital discharge (J Am Coll Cardiol 2017;69[17]:2105-15).
As the NEJM editorial put it: “Antiarrhythmic drugs and catheter ablation can reduce VT recurrence rates, but both treatments have limited efficacy and important side eff ects.”
SBRT offers what might be called a kinder, gentler approach to restoring normal heart rhythm. “What we’ve developed is a way to do a complex heart ablation entirely noninvasively in about seven to 10 minutes,” emphasizes Cuculich. “And when the procedure is over, the patient gets up from the table, walks out and goes home. Compare that to the standard of care, which would have them in the hospital for one to three days, oftentimes in intensive care, and facing a potentially long recovery once they get home.”
 HOW SBRT WORKS
HOW SBRT WORKS
Catheter-free, electrophysiology-guided ablation for VT represents a fusion of two techniques: noninvasive mapping of cardiac arrhythmias through electrocardiographic imaging, and noninvasive delivery of precise ablative radiation through SBRT.
While training as a cardiology fellow at Washington University, Cuculich began thinking about noninvasive treatment for arrhythmias. Whetting his interest was the close collaboration he developed with his research mentor, Yoram Rudy, PhD, who joined Washington University in 2004 and founded the interdisciplinary Cardiac Bioelectricity and Arrhythmia Center (CBAC), which today numbers nearly 40 faculty members. Rudy invented noninvasive electrical mapping, which paved the way for Cuculich and other innovators to develop electrocardiographic imaging to pinpoint the source of arrhythmias emanating from the lower chambers of the heart.
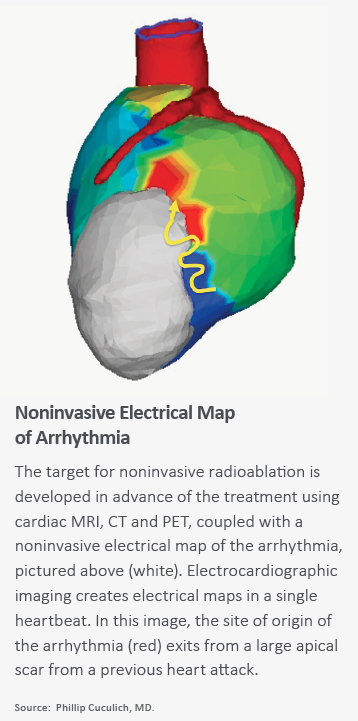
The electrical mapping part of the invasive procedure is intense and time-consuming compared to noninvasive cardiac mapping. Pictures of the heart are taken through standard imaging: MRI, CT, PET scans. But the noninvasive electrocardiographic mapping that follows is unique. Wearing a vest of 256 electrodes (vs. 10 for most electrocardiograms), patients are brought into the electrophysiology lab and undergo noninvasive programmed stimulation with the use of an indwelling implantable cardioverter defibrillator to induce VT. When data from this electrocardiographic imaging map are overlaid with the standard cardiac imaging, physicians get a clear and comprehensive picture of both the myocardial scar and the arrhythmogenic region from which the ventricular arrhythmias are originating. Just as important, this novel mapping allows them to determine where the pathology is headed.
The intricate maps also enable the second part of the procedure: precise delivery of a highly focused dose of radiation (comparable to what a patient with early-stage lung cancer might be given) that targets the body with minimal damage to normal adjacent tissue. “We do everything we can to ensure that dose of radiation is closely associated with only the diseased parts of the heart,” emphasizes Robinson, “because we know that if we start treating patients with a longer projected life span, this could become a real issue for them.”
Cardiac Electrophysiology Society President Curtis draws a distinction between standard radiation therapy for cancer and for ablation. The former, she points out, increases patient exposure by requiring a typical regimen of six weeks of treatment, five days a week. But with SBRT, she says, “we’re talking about a single procedure with pinpoint accuracy to ablate specific circuits. I would therefore think it’s unlikely that the radiation itself is going to have long-term issues.”
ON THE HORIZON
Experts in arrhythmia treatment and management are hopeful SBRT could provide a springboard for more innovation in a field long overdue for change. “We are now looking for the next step after VT ablation, where we can do it in a much more simplified manner with less risk and hopefully greater effectiveness,” says John.
One such approach—known as intramural needle ablation catheter (INA)—was developed by William Stevenson, MD, who is John’s colleague at Vanderbilt University Medical Center and his coauthor on the NEJM editorial. INA is designed to address the fact that some people have ventricular arrhythmias that originate deep inside the heart muscle, outside the range of standard catheter ablation. The safety and efficacy of the needle ablation catheter for locating and ablating ventricular arrhythmias that have failed standard radiofrequency ablation is the focus of a 50-patient clinical trial set to conclude in September 2018.
Another experimental technique for treating VT is saline enhanced radiofrequency ablation (SERF). Still in early-stage development, SERF uses radiofrequency electrical energy delivered through a catheter-type needle to inject warm saline into the tissue, overheating and killing scarred myocardial residue.
As some practitioners see it, however, the most exciting prospects are actually outside the realm of ablation, on the prevention side. Robinson and Cuculich, for example, cite research underway in areas like gene modification and attempts to build back normal heart tissue as a way of preventing the onset of arrhythmia.
SBRT continues to be fair game for innovation, as researchers and clinicians work to improve its electrical mapping component to more precisely determine the location of a patient’s heart scars. They also are considering ways to improve the delivery of focused radiation and to better understand the actual biological effect of radiation on the heart. This, in turn, could potentially lead to new techniques for desensitizing tissue to radiation or to eliminating arrhythmias without damaging nearby organs and tissue.
Opportunities to refine these and other cutting-edge ideas and techniques are plentiful, reports Cuculich, whose team is talking to some 30 research centers around the world that have ex-pressed an interest in being part of the next SBRT clinical study. “What gives us the most excitement is the fact we’ve come up with a safer, faster experience for patients with ventricular tachycardia—and with very encouraging results,” Cuculich says. “Now, it’s up to us to carefully study the longer-term risks and the potential of our approach to really understand where noninvasive ablation fits into our armamentarium.”
