To conquer STEMI’s ‘last frontier,’ cardiologists are tapping into an evolving arsenal of strategies while calling for more data and standardized definitions to guide treating physicians.
Thanks to significant advances in cardiac procedures and protocols over the past decade, deaths from heart attack have dropped dramatically. The best example is the 21 percent mortality reduction that followed the use of percutaneous coronary interventions (PCIs) in patients with acute myocardial infarction (J Am Coll Cardiol 2015;8:139-46).
But one area has stubbornly resisted the tides of change, locked in for decades at a nearly 50 percent in-hospital mortality rate (J Am Coll Cardiol 2016;67:1881-4). That area is acute myocardial infarction complicated by cardiogenic shock (AMI-CS). Out of frustration, puzzlement or pique, some cardiologists have labeled it STEMI’s “last frontier,” while Twitter debate on the subject has coined #WarOnShock.
Each year, about 60,000 patients—approximately 8 percent of patients with AMI—suffer cardiogenic shock, presenting physicians with tense, life-or-death choices that play out in real time (Circulation 2017;135:e146-603). The problem is that those choices are seldom obvious or well-defined, and no consensus has evolved on the best clinical path forward for saving lives. It speaks volumes that cardiologists still don’t have a clear definition of cardiogenic shock or understanding of its pathophysiology to help them determine which patients have entered that advanced state. Moreover, clinical trials for the various mechanical circulatory support (MCS) devices on the market have added to the confusion over the most effective approach to treatment.
“There’s this mindset that you just open a vessel, tuck the patient into the intensive care unit and then wait and see how they are the next morning,” says Alexander Truesdell, MD, co-director of the INOVA Heart and Vascular Institute’s Cardiogenic Shock Initiative in Virginia. Truesdell, a former U.S. Army physician who served in Iraq, Afghanistan and the Balkans, has called for a nationwide “combat approach” to cardiogenic shock comparable to what the military has employed for the past 20 years to revolutionize the management of battlefield polytrauma toward a goal of zero preventable deaths.
There are signs suggesting the war on cardiogenic shock may be entering an urgent new phase. Some tertiary care hospitals around the country have created multidisciplinary teams of cardiothoracic surgeons, interventional cardiologists, advanced heart failure specialists and allied health professionals that can be activated quickly when a patient with possible cardiogenic shock presents to the emergency department. A few health systems are even building hub-and-spoke networks of hospitals, analogous to trauma care, for treating STEMI patients complicated by cardiogenic shock.
 Meanwhile, the growth of MCS devices that are used during high-risk revascularization to maintain adequate perfusion and prevent irreversible end-organ damage has given the field a promising, if still unproven, tool. These devices include the intra-aortic balloon pump (IABP), still the most widely used MCS in cardiogenic shock; the Impella (Abiomed) family of devices that primarily unload the leftventricle and stabilize the patient’s hemodynamics; the Tandem Heart (LivaNova), another percutaneous left ventricular assist device; and the extracorporeal membrane oxygenator (ECMO), which increases left ventricular systolic and diastolic pressure and peripheral perfusion but does not unload the ventricle.
Meanwhile, the growth of MCS devices that are used during high-risk revascularization to maintain adequate perfusion and prevent irreversible end-organ damage has given the field a promising, if still unproven, tool. These devices include the intra-aortic balloon pump (IABP), still the most widely used MCS in cardiogenic shock; the Impella (Abiomed) family of devices that primarily unload the leftventricle and stabilize the patient’s hemodynamics; the Tandem Heart (LivaNova), another percutaneous left ventricular assist device; and the extracorporeal membrane oxygenator (ECMO), which increases left ventricular systolic and diastolic pressure and peripheral perfusion but does not unload the ventricle.
As these devices get more attention, access to them is still limited to a few centers. According to the National Cardiovascular Data Reg-istry, MCS is used in just 3.1 percent of AMI-CS cases (J Am Coll Cardiol 2017;69:1427-50). In a review article titled “Cardiac Shock Care Centers,” William O’Neill, MD, of Henry Ford Health Care System, and coauthors stressed that clinical trials have failed to clarify the best treatment strategy and “no standardized agreement on the utilization of mechanical circulatory support” has emerged to resolve what they referred to as “a national issue” (J Am Coll Cardiol 2018;72:1972-80).
Indeed, the oft-cited IABP-SHOCK II trial demonstrated no benefit for IABP in the management of AMI-CS patients. Meanwhile Richard Smalling, MD, PhD, says the CRISP AMI study, based on positive animal studies, paints a different picture. “We found that left ventricular unloading with the balloon pump prior to reperfusion in STEMI patients with large anterior infarcts but without shock actually resulted in a 70 percent reduction in heart failure, cardiogenic shock and death at six months compared to not using the pump,” says Smalling, who is professor and chair of cardiovascular medicine at UT Health/Memorial Hermann Heart and Vascular Institute in Houston. In an interview with Cardiovascular Business, he said his take-home message at TCT.18, where he discussed CRISP AMI, was this: “It takes two minutes to put a balloon pump in, and physicians should consider doing so prior to performing PCI in patients with large anterior infarcts, especially in those with early hemodynamic compromise.”
Assessing MCS effectiveness
Some in the field are pushing for a large randomized controlled trial (RCT) that could provide expert guidance. William Suh, MD, an interventional cardiologist at the David Geffen School of Medicine at UCLA, has been outspoken on Twitter, insisting that without an RCT physicians can’t be certain they’re helping patients when using Impella. “We need a properly conducted trial to provide sufficient evidence Impella is safe and effective in AMI-CS,” Suh told CVB, noting that such a large-scale RCT “does not seem possible in the U.S. any longer [because] physicians here are too biased and are unlikely to enroll patients.” Instead, he believes an RCT should be undertaken in a country that doesn’t yet have the device.
In fact, a European trial is underway. Launched in 2012, the randomized multicenter DAN-GER Trial (Effects of Advanced Mechanical Circulatory Support in Patients with ST Segment Elevation Myocardial Infarction Complicated by Cardiogenic Shock) plans to enroll 360 patients and randomize them to either conventional circulatory support or the Impella device. Other medications such as concomitant inotropic support can be used as needed in either arm. The estimated study completion is January 2023. Other prospective observational efforts include theNational Cardiogenic Shock Initiative, which is testing a best practices algorithm for treatment of AMI-CS patients (Catheter Cardiovasc Interv 2018;91[3]:454-61).
Even without large-scale studies, many experts see an important role for MCS devices going forward. “Anecdotally and observationally, we believe they are ultimately needed as a bridge to recovery or a bridge to transplant,” says Srihari S. Naidu, MD, director of the cardiac catheterization laboratory and the Hypertrophic Cardiomyopathy Center at Westchester Medical Center in New York. Naidu helped organize “SCAI SHOCK,” a Society for Cardiovascular Angiography and Interventions (SCAI) course that drew 350 participants to its first training session in an effort “to move the needle on our understanding and treatment of cardiogenic shock.”
With regard to MCS, Naidu adds, “Even though there’s not a lot of data, it’s very clear there is no way around using these devices in trying to improve mortality in [cardiogenic shock] patients.” He is working with a host of professional societies and groups to develop a comprehensive definition of cardiogenic shock with clear clinical, biochemical and hemodynamic signs. The resulting nomenclature is intended to help physicians know for the first time where on the shock spectrum individual patients reside and to assess whether they are improving or deteriorating. That consensus document is expected to be published in 2019.
A new approach
The next big leap in the battle against AMI-CS likely may be through comprehensive regional networks of smaller hospitals linked to large centers of excellence that are equipped to treat all levels of cardiac shock with team-based, multidisciplinary care and rapidly deployable percutaneous MCS. In their 2018 review article, O’Neill and coauthors spelled out a framework for such an approach, patterned after the successful national system for trauma care. Acknowledging that the cost and complexity of advanced support is prohibitive for smaller hospitals where procedural volumes are small, they recommended three levels of care:
- Level I: Dedicated shock care centers with cardiac catheterization and advanced MCS delivered through “cardiogenic shock teams” of highly skilled specialists
- Level II: Hospitals with PCI but no advanced MCS that are prepared for rapid transfer of shock patients to Level I centers
- Level III: Mostly rural hospitals with no PCI capability but a written plan for emergent transfer to a dedicated shock care center
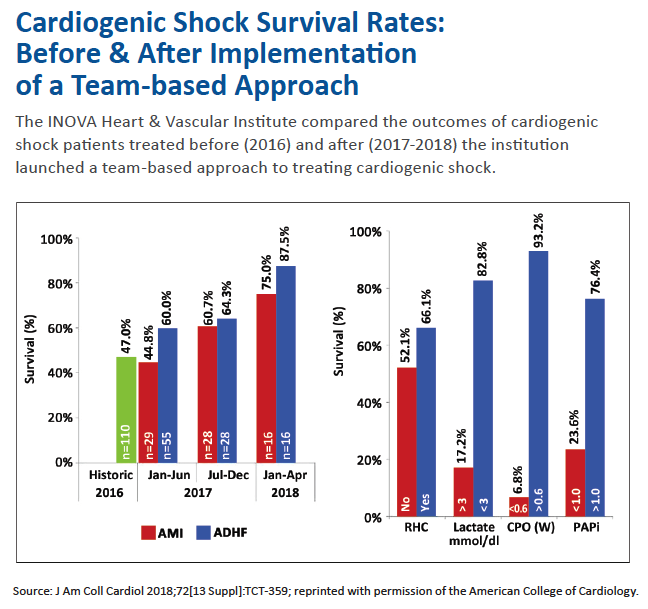
That comprehensive hub-and-spoke approach is already achieving impressive results under INOVA Heart and Vascular Institute’s Cardiogenic Shock Initiative. If shock is suspected in an AMI patient, a single phone call by a physician anywhere within INOVA’s system to a 24/7 operator results in the immediate convening of a five-member team of cardiac shock specialists—either at bedside or telephonically—for a brief consultation and consensus plan of action.
“You realize how much good thinking can emerge from just a quick group discussion of the situation and sharing of expertise,” says Truesdell. Also critical to the approach is constant assessment, adjustment, reassessment and readjustment—a schema Truesdell refers to as the “unblinking eye,” which has worked well on the battlefield.
As for INOVA’s bold initiative, more than 240 patients with a diagnosis of cardiogenic shock have been treated from the start of the program in January 2017 to date. The survival rate rose from 47 percent in 2016 to 58 percent in 2017 to 77 percent through June 2018 (J Am Coll Cardiol 2018;72[13 Suppl]:B146[TCT-359]).
Those findings, presented at TCT.18, have left Truesdell more convinced than ever of the potential rewards from a combat approach to cardiogenic shock. “We have to change what we’ve been doing and organize around large, centralized centers of excellence with highly specialized multidisciplinary shock teams,” he asserts. “Patients must be identified earlier and advanced care—often to include mechanical circulatory support—delivered more rapidly. Only by employing our resources in a very aggressive but targeted way can we halt what’s been persistent and unacceptably high mortality for cardiogenic shock.”
Editor's Note: This story was updated to reflect that the DAN-GER trial is being conducted in Europe.
