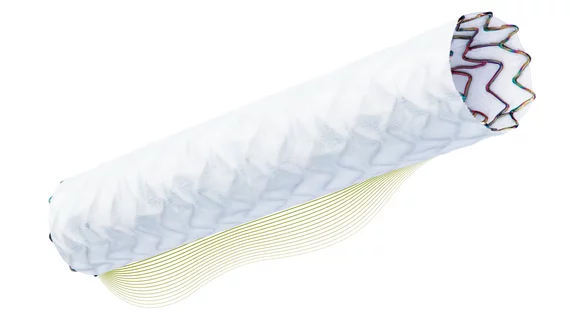
The FDA has approved the PK Papyrus covered coronary stent system to treat acute coronary artery perforations, device maker Biotronik announced Sept. 14. It is the first device to be approved by the FDA for this indication in 17 years.
“An acute coronary artery perforation is a rare, but potentially life-threatening complication of heart vessel procedures,” Bram Zuckerman, MD, director of the Division of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in a statement. “The PK Papyrus Covered Coronary Stent System provides health care providers with a new treatment option that can seal the perforation in order to stop blood leakage during the procedure and avoid a potentially life-threatening complication or a more invasive surgical procedure.”
According to the FDA, a deep tear in a vessel wall can occur during percutaneous coronary intervention (PCI), after which blood may leak and pool dangerously in the sac surrounding the heart.
The PK Papyrus system can be implanted in a perforated artery with a balloon catheter similar to one used in a typical PCI procedure. The difference is the covered stent provides a physical barrier to seal the tear in the artery while still allowing blood to flow through the device.
"In rare cases of a coronary perforation, time is the enemy," Dean Kereiakes, MD, an interventional cardiologist and medical director at the The Christ Hospital and Vascular Center in Cincinnati, said in Biotronik’s release. "The device's superior flexibility and tracking means that PK Papyrus delivers more like a conventional stent and can treat a perforation more quickly, avoiding further complications. Hospitals need to have this potentially lifesaving device ready for physicians to use."
The FDA granted the approval through its humanitarian device exemption process, which is reserved for devices that are intended to benefit patients for a condition that affects less than 8,000 Americans per year. Fewer than 4,000 PCIs in the U.S. each year require a covered stent, according to the release.
PK Papyrus is available in 17 sizes, extending the ability to treat perforations to vessels between 2.5 and 5 millimeters in diameter. It is the only 5 French compatible coronary covered stent available in the U.S., Biotronik said.