Is cardiac PET a game-changer for cardiac imaging? Could myocardial perfusion PET eventually assume the role of SPECT? For some cardiology constituents, the answer is yes, based on a compelling clinical case that includes a growing number of studies. For example, in 2012, researchers who reviewed 15 cardiac SPECT and PET studies found that PET trumped SPECT on both sensitivity (90 percent vs. 85 percent) and specificity (88 percent vs. 85 percent) (J Am Coll Cardiol 2012;60:1828-37). And in another study, cardiac PET slashed invasive coronary arteriography and coronary artery bypass graft surgery rates by 50 percent in patients with intermediate pre-test likelihood of coronary artery disease (J Nucl Med 2007;48:1069-76).

“There is no question that cardiac PET is the next step clinically, based on high diagnostic accuracy, consistent image quality, low radiation exposure, short acquisition protocols, quantifi cation of myocardial blood flow and risk stratification,” says Vasken Dilsizian, MD, professor of radiology and medicine at the University of Maryland and chief of nuclear medicine at the University of Maryland Medical Center.
Still, Dilsizian emphasizes, cardiac PET “also has to be feasible from a financial and logistical standpoint.” The nonclinical requirements, he and other sources say, help explain the relatively low number of cardiac PET programs in the U.S.—approximately 200, according to industry estimates.
Do the math
Of all the issues that merit exploration before cardiologists invest in myocardial perfusion PET, equipment selection and cost parameters top the list.
While the “set of applications” a provider is considering—for example, standalone cardiac PET or hybrid PET-CT—determines the type of equipment required for its cardiac PET program, almost all camera manufacturers now offer only hybrid PET-CT systems, says Suresh Kuppuswamy, MD, principal medical imaging analyst at Frost & Sullivan, a consulting firm in Mountain View, Calif.
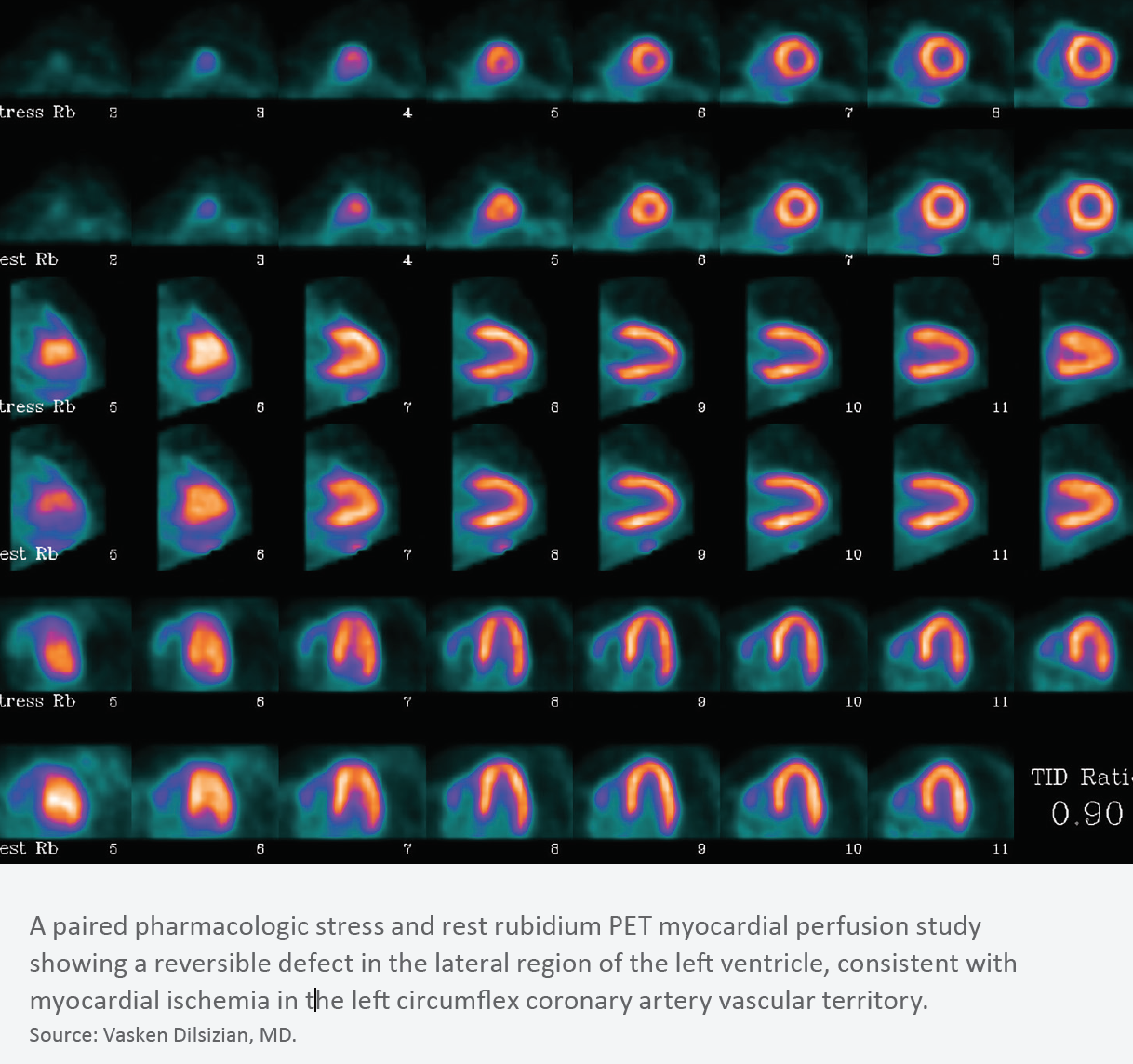 Providers who prefer standalone cardiac PET will be limited to refurbished systems available in the marketplace, opening the door for problems down the road. “The risk lies in servicing, as parts for a refurbished system can become unavailable anytime in the future,” Kuppuswamy explains.
Providers who prefer standalone cardiac PET will be limited to refurbished systems available in the marketplace, opening the door for problems down the road. “The risk lies in servicing, as parts for a refurbished system can become unavailable anytime in the future,” Kuppuswamy explains.
Practices should expect to spend between $350,000 and $450,000 for a refurbished system, plus around $85,000 per year for maintenance, according to Kuppuswamy and other sources. A new PET-CT system will run upwards of $1.5 million, with cardiology practices and hospitals incurring an additional 10 to 12 percent of the equipment cost toward maintenance each year.
Space and shielding requirements will add to the tab. If PET-CT is to be an adjunct to the SPECT system, Kuppuswamy says the budget must cover the construction or renovation of a 14x24-foot room or area to accommodate the equipment, generator with storage cart, infusion system and control section as well as give technologists sufficient room to move.
Ongoing monetary outlay for radiopharmaceuticals is another cardiac PET expenditure worth examining before committing. Marcelo F. Di Carli, MD, director of cardiovascular imaging and chief of nuclear medicine and molecular imaging at Brigham and Women’s Hospital in Boston, says rubidium-82 is a more practical radiopharmaceutical option than N-13 ammonia for PET myocardial perfusion imaging in part because rubidium-82 is eluted in a portable generator and, unlike N-13 ammonia, does not require cyclotron production.
Regardless of the number of doses eluted, all rubidium generators must be replaced often. Monthly, according to Kuppuswamy. At most every six months, Dilsizian says. Each generator replacement costs $20,000 to $30,000, resulting in fixed annual costs of approximately $120,000 to $432,000 (depending on the exact price per generator and the frequency of replacement).
Compared to SPECT (MBI) radiotracers, which sources say can be obtained at a cost of $50 to $100 per dose, “rubidium-82 achieves a break-even only when the number of patients for cardiac PET hits the seven to eight cases per day mark,” Kuppuswamy says.
Cardiology practices and hospital cardiology departments will always need to thoroughly examine whether their pockets are deep enough to support the equipment investment and radiopharmaceuticals-related expenditures inherent in introducing cardiac PET. However, equipment prices may become less of a factor in determining whether or not to proceed. “Right now, finding new PET cameras that cost less than $1 million is challenging,” says Di Carli, who has studied the efficacy and benefits of cardiac PET. “However, manufacturers are working on building cardiology-specific PET cameras that command a lower price.”
Another potential bright spot is the second phase III clinical trial examining whether flurpiridaz (Lantheus) 18F injection might be an alternative to currently approved radiopharmaceuticals in PET MPI. The AURORA trial involves 650 subjects with suspected coronary artery disease, all of whom will undergo SPECT MPI and flurpiridaz 18F injection prior to coronary angiography. Final follow-up is slated for August 2020.

“The object is to look at the diagnostic efficacy—sensitivity and specificity—of flurpiridaz 18F injection,” Di Carli explains, “and the likelihood is that the news will be positive.” Also promising is that the isotope can be delivered in unit doses, potentially making a cardiac PET program more financially feasible for providers, he adds.
Finally, for cardiology providers looking to offer both SPECT and PET imaging, the possibility of shrinking SPECT revenues merits careful consideration. “Payment reform—aka value vs. volume—dictates that the nuclear laboratory may become a cost center,” observes Randal White, MD, of the Cardiology Clinic of San Antonio-Medical Center, which is in the process of launching a cardiac PET program. “In other words, services are included in the price. PET, CT and MRI, while complementary, will likely become competitive technologies as healthcare dollars dry up.”
Kuppuswamy agrees. “Parallel operation of SPECT and PET-CT systems will result in a loss of revenues and margins for the SPECT program because of cannibalization by the PET-CT program,” he predicts.
It’s critical to assess the likelihood that anticipated increased patient volume and revenues generated by cardiac PET will be sufficient to offset the cannibalization effect, White and Kuppuswamy say. There’s no way to know for certain, but a litmus test is whether your cardiac PET program will serve at least eight patients per day—ideally more—to compensate for inevitable attrition from the SPECT side.
Catering to certain patient populations may mitigate the impact of SPECT losses, rendering it easier to justify a new cardiac PET program. For example, Cardiology Clinic of San Antonio-Medical Center has a large volume of patients who are unable to complete a diagnostic-level exercise stress imaging study. In a 2016 position statement on the clinical indications for myocardial perfusion PET, the American Society of Nuclear Cardiology and the Society of Nuclear Medicine and Molecular Imaging classified PET as the “preferred test” for such patients (J Nucl Cardiol 2016;57:1654-6).
“In our practice, such a patient scenario accounts for approximately 60 percent of SPECT studies,” White notes. With implementation of a new PET program, he says, “pharmacological SPECT studies will thus become pharmacological PET studies—a large number that makes the venture viable for our practice and, I suspect, most others.”
Consider C-suite concerns
Building the business case for cardiac PET might include addressing questions from the C-suite about reimbursement, such as limitations that have made some administrators hesitant.
“Cardiac PET is being reimbursed in some states, but not in others, and sometimes it’s even more complex than that,” Di Carli says. For example, in late 2018, BlueCross BlueShield of Tennessee began covering cardiac PET for patients with either significant left ventricular dysfunction under consideration for revascularization or suspected cardiac sarcoidosis. However, the payer classifies cardiac PET to determine a bsolute quantification of myocardial blood flow as “investigational” and doesn’t cover it.
Meanwhile, the Centers for Medicare & Medicaid Services (CMS) reimburses FDG PET only for patients with coronary artery disease and left ventricular dysfunction and in cases where it is used in tandem with myocardial perfusion imaging to identify left ventricular myocardium with residual glucose metabolism and possible reverse loss of systolic function.
If reimbursement concerns are holding back C-suite buy-in, Di Carli advises pointing out that “although reimbursement for cardiac PET is not all-encompassing and is only slightly better than for SPECT, it gives practices and service lines a unique differentiator to compete in the marketplace” and to generate increased patient volume. “Things may balance out,” he says.
It also might help to bring to the table other applications for cardiac PET equipment—and hence, opportunities to increase patient volume and revenue streams. Dilsizian and Di Carli emphasize cardiac PET’s value for evaluating implantable cardioverter-defibrillator infections (J Am Coll Cardiol 2012;59:1616-25), prosthetic valve infections, cardiac sarcoidosis (J Nucl Cardiol 2019;26[1]: 188-99) and possibly graft infections in CABG patients.
The hospital partners of Cardiology Clinic of San Antonio-Medical Center are purchasing a refurbished CT-PET scanner, says White. Their plan is not only to use rubidium via onsite generator but also to design and shield the lab for multiple F18 agents. These include flurpiridaz as well as FDG (which is presently reimbursed for viability studies and, he says, will “hopefully soon be reimbursed widely for sarcoid studies”), along with agents being investigated for amyloid evaluation.
“We are a large group with more than 30 practicing cardiologists, so the actual numbers to make a rubidium PET program financially viable were self-evident,” White says. “The larger question was whether or not to request the money required for additional finish-out to allow for F18 imaging.” To make the case, White outlined future prospects for sarcoid imaging and for F18, including amyloidosis, various inflammatory/infectious disease states and “the present approved use in myocardial viability imaging,” he explains. “My hope was to broaden horizons beyond simple perfusion imaging. So far, so good.”