Cardiac Surgery
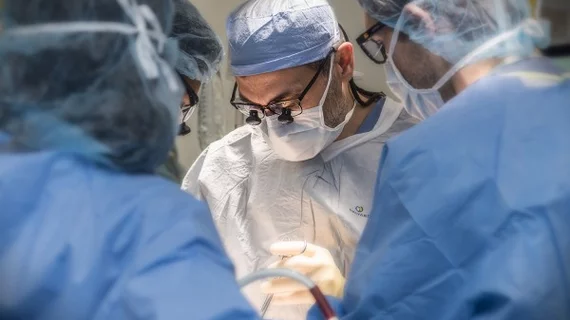
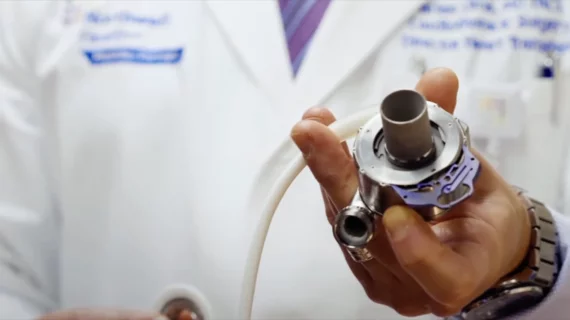
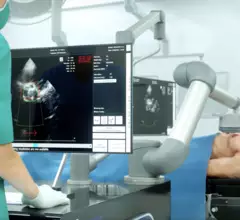
Cardiothoracic surgery includes coronary artery bypass surgery (CABG), heart valve repair or replacement, left ventricular assist device (LVAD) placement, heart transplant, assisting in minimally invasive transcatheter valve structural heart procedures such as TAVR, left atrial appendage (LAA) occlusion, septal myectomy, surgical ablation for arrhythmias, and reconstruction of the heart in congenial heart disease cases.
Displaying 49 - 56 of 215